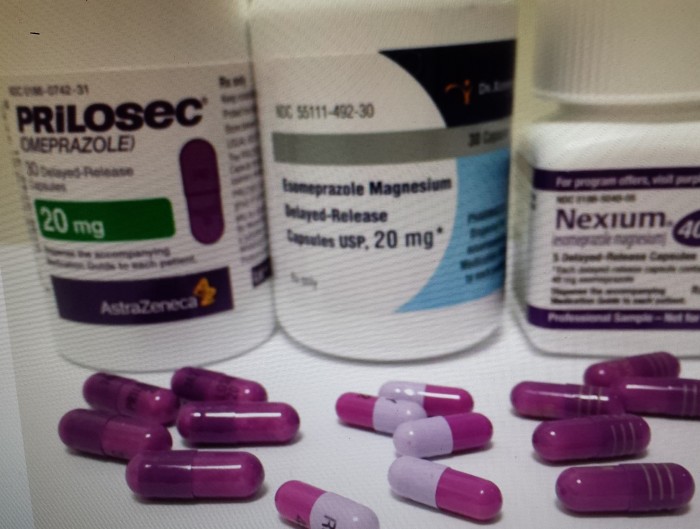
Americans are spending more on prescription drugs than in other countries
By Ed Silverman
Rising costs for prescription drugs are straining budgets around the globe. The 34 countries in the Organization for Economic Cooperation and Development, which includes the United States, spent $800 billion on pharmaceuticals in 2013, a new report finds. That’s a full 20 percent of total health spending.
And here's one key finding: the US spent twice the OECD average on pharmaceuticals purchased at retailers in 2013. The group average was about $500 per person. In the United States, it was $1,026 per person. Read More